Abstract
Background: Patients with cancer have an increased risk of developing atrial fibrillation (AF). Patients with cancer who develop AF have an increased risk of cardiovascular-related death at 12-months. Use of anticoagulant therapy may benefit these patients. However, patients with cancer are at increased risk of anticoagulant-related bleeding. Several risk prediction models are available to estimate the risk of anticoagulant-related bleeding in patients with AF. However, these models were developed predominately in patients without cancer. Thus, the utility of these models in cancer is unknown. We aimed to evaluate the performance of existing bleeding risk models in a cohort of patients with active cancer and AF starting on anticoagulant therapy.
Methods: Using a nationwide cohort of US Veterans, we identified patients with active cancer and AF with a new prescription for anticoagulant therapy between 2012 and 2018. Cancer was defined as active if the diagnosis of AF occurred within 3 months prior to up to 6 months after new cancer diagnosis or in the setting of incurable hematological cancers (e.g., multiple myeloma, chronic myelogenous leukemia) or metastatic solid tumors. We identified anticoagulant-related major bleeding (MB) within 6 months of anticoagulant start by the presence of inpatient ICD-9/10 codes in any position as previously validated. Patients were censored 30-days after last anticoagulant prescription. We evaluated the predictive performance of six models. The association per standard deviation increase in the score and MB within 6 months of anticoagulant start was estimated using Fine-Gray subdistribution survival analysis to account for the competing risk of death≥÷A sensitivity analysis limiting inpatient ICD-9/10 codes for MB to the first two positions was also performed. We evaluated model discrimination using Harrell's c-statistic.
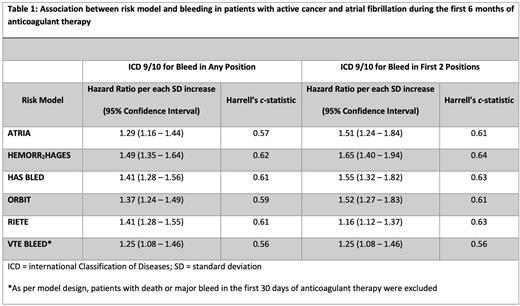
Results: 5716 patients met inclusion criteria into the cohort. The most common types of cancer included: hematological malignancies (24%), lung (20%), prostate (18%), non-prostate genitourinary (11%), and gastrointestinal (10%) cancer. Mean age at the start of anticoagulant therapy was 73.4 years and median overall survival 41.1 months. Of the cohort, anticoagulant therapy was as follows: 2040 warfarin, 179 LMWH, 5 fondaparinux, and 3495 DOAC. 328 (5.7%) patients had a MB within 6 months of anticoagulant start and 439 (7.7%) within 12 months. The most common type of bleeds within the first 6 months of anticoagulant therapy was gastrointestinal (47%), followed by hematuria (28%) and hemoptysis (13.7%). 2.4% of bleeding events (n=8) were intracranial hemorrhage. The increase in hazard of bleeding per each standard deviation increase in bleeding score is in Table 1. For model discrimination, Harrell's c-statistic for each score is also listed in Table 1.
Conclusions: In this cohort of 5716 patients with active cancer and atrial fibrillation starting on anticoagulant therapy, available clinical risk models moderately predicted bleeding. The addition of cancer-specific risk factors might improve accuracy of the available models.
Disclosures
Sanfilippo:ACS-IRG: Research Funding; Covington & Burling LLP: Other: Expert Case Review; Health Services Advisory Group: Consultancy; K01 NHLBI: Research Funding; NHLBI NIH: Other: Loan Repayment program; Quinn Johnston: Other: Expert Case Review.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal